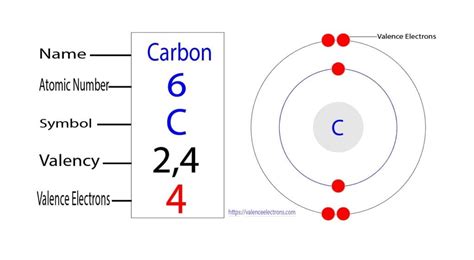
carbon valence electrons|2.9: Valence Electrons : Pilipinas In methane, carbon has a valence of 4; in ammonia, nitrogen has a valence of 3; in water, oxygen has a valence of two; and in hydrogen chloride, chlorine has a valence of 1. .
If we look at the element after nitrogen in the same period, oxygen (Z = 8) its electron configuration is: 1s 2 2s 2 2p 4 (for an atom). Oxygen has one more electron than nitrogen and as the orbitals are all half filled the electron must pair up. . O (Z=8) configuration:1s 2 2s 2 2p 4; F (Z=9) configuration:1s 2 2s 2 2p 5; Ne (Z=10) .
carbon valence electrons,Mar 23, 2023 Carbon has two valence electrons, which means it can form bonds with two .2.9: Valence Electrons Valence electrons are also responsible for the bonding in the pure chemical elements, and whether their electrical conductivity is characteristic of metals, semiconductors, or insulators. Metallic elements generally have high electrical conductivity when in the solid state. In each row of the periodic table, the metals occur to the left of the nonmetals, and thus a metal has fewer possible valence electrons than a nonmetal. However, a valence electron of a metal atom has .

Learn how to determine the number of valence electrons for main group elements using the periodic table. See patterns, examples, and tips for identifying valence electrons.
Valence shell electrons (or, more simply, the valence electrons) are the electrons in the highest-numbered shell, or valence shell, while core electrons are the .
In methane, carbon has a valence of 4; in ammonia, nitrogen has a valence of 3; in water, oxygen has a valence of two; and in hydrogen chloride, chlorine has a valence of 1. .

Learn how the periodic table reflects the number and pattern of electrons in atoms, and how this determines their chemical reactivity. Find out how to use the Bohr model and .carbon valence electronsLearn how the periodic table reflects the number and pattern of electrons in atoms, and how this determines their chemical reactivity. Find out how to use the Bohr model and .
carbon valence electrons 2.9: Valence Electrons Valence electrons are the electrons that reside in the outermost energy level of an atom and are, therefore, the most accessible for the formation of chemical .
carbon valence electrons|2.9: Valence Electrons
PH0 · What Are Valence Electrons? Definition and Periodic
PH1 · Valences of the Elements Chemistry Table
PH2 · Valence electrons and ionic compounds (video)
PH3 · Valence electron
PH4 · Valence Electrons Chart for All Elements
PH5 · The periodic table, electron shells, and orbitals
PH6 · Determine valence electrons using the periodic table
PH7 · 2.9: Valence Electrons
PH8 · 10.6: Valence Electrons
PH9 · 1.3: Valence electrons and open valences